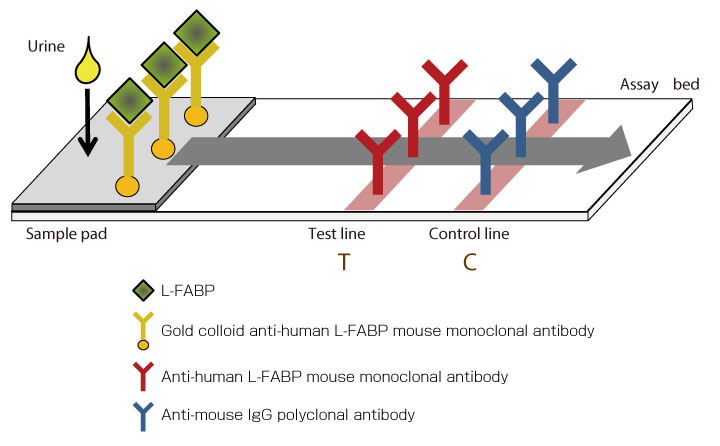
Measurement principle
POC
RENISCHEM® L-FABP POC Kit is an immunochoromato-graphic test for detection and semi-quantitative determination of L-FABP. Urinary L-FABP levels can be visualized within 15 minutes after the specimen is added to the sample pad. L-FABP in the urine samples reacts to a gold colloid anti-human L-FABP mouse monoclonal antibody (CloneL), forms an antigen-antibody complex, move through the membrane by a capillarity action, and reacts to the anti-human L-FABP mouse monoclonal antibody on the Test Line (Clone2), visualizing a burgundy color. RENISCHEM® L-FABP POC Kit allows semi-quantitative measurement by comparing the color density of Test Line with that on reference card.
Additionally, the gold colloid anti-human L-FABP mouse monoclonal antibody (CloneL) moves further on the membrane and binds with the anti-mouse IgG polyclonal antibody on the Control Line, visualizing a burgundy color. This line is an index for checking that the test is performed properly.
Urinary L-FABP level displayed on testline

Determining results of measurement
When a line appears on the Control Line, read the color intensity of the Test Line and make an assessment comparing with three levels of concentration printed on a reference card.
ELISA method
RENISCHEM®ELISA test is a kit based on enzyme immunoassay (ELISA method) for fixed-quantity L-type fatty acid binding proteins (L-FABPs) in human urine.

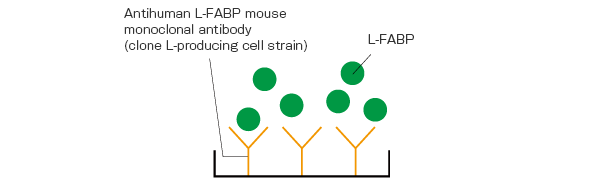
After processing with Pretreatment Solution, standard L-FABPs or urine samples are made to react by adding them to anti-L-FABP antibody coated microplate into which Assay Buffer has been dispensed. The L-FABPs in the Assay Buffer bind to the Antibody-coated Microplate.

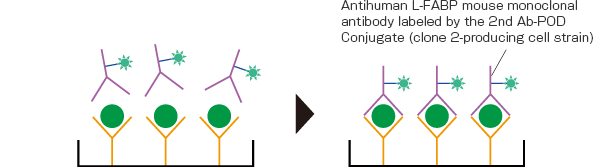
By washing the plate and inducing a reaction by adding the 2nd Ab-POD Conjugate as secondary antibodies, a sandwich-bound substance corresponding to the amount of L-FABP is formed from the solid-phased antibody – antigen – antibody labeled by the 2nd Ab-POD Conjugate.

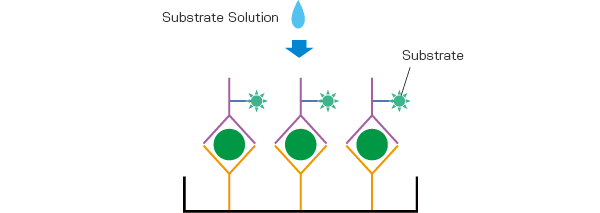
After that reaction, by washing and inducing an enzyme reaction by adding Substrate Solution, coloring corresponding to the amount of L-FABP antigen present can be observed. The L-FABP concentration is found by measuring the absorbance using a microplate absorptiometer and creating an analytical curve based on the obtained absorbance.
Datermining results of measurement
The urinary L-FABP concentration (ng/mL) obtained from the analytical curve based on the sample’s absorbance should be corrected with urinary creatinine levels. The standard level of urinary L-FABP : Creatinine ratio is 8.4μg/gCr or less.
References
- [1] Lucchese, B. et al., Diabetes: A new urinary marker predicts progression to albuminuria and risk of death in patients with type 1 diabetes mellitus. Nat Rev Nephrol. 6(8): 445, 2010. PubMed
- [2] Kamijo-Ikemori, A. et al., Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care. 34(3): 691-696, 2011. PubMed
- [3] Doi, K. et al., Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med. 39(11): 2464-2469, 2011. PubMed