About L-FABP
FAQs
L-FABP
Samples, measurement
Product performance/operation
Research use
-
Q1What is L-FABP?
-
A1L-FABP stands for “L-type Fatty Acid Binding Protein” and is a low molecular weight soluble protein (14 kDa) that is specifically expressed in the liver and renal proximal tubules. Because L-FABP is a substance derived from the cytoplasm of the renal proximal tubules and is excreted in urine due to renal tubular ischemia and oxidative stress on renal tubules, urinary L-FABP is useful for early diagnosis of renal disease accompanying tubular dysfunction.
-
Q2Is liver-derived L-FABP excreted in urine?
-
A2Usually L-FABP excreted by liver is reabsorbed by kidney in healthy condition and it is not or almost not excreted in urine. However, when liver origin L-FABP is no longer reabsorbed by the kidney, it is excreted in urine.
In the case of the fulminant hepatitis and hepatorenal syndrome, approximately 12% of urinary L-FABP is from the liver, but liver-derived L-FABP can be easily distinguish from kidney-derived L-FABP by using in combination with other biomarkers. -
Q3How is L-FABP different from urinary albumin?
-
A3Urinary albumin is a glomerular injury marker that indicates the result of filtration failure. Its quantity excreted in the urine increases with the progression of the glomerular injury. In contrast, L-FABP is a marker reflecting tubular dysfunction, and is excreted in the urine in response to ischemia and oxidative stress.

-
Q4What is the clinical utility of L-FABP?
-
A4If you are interested in the clinical utility of L-FABP, please click here. Clinical Utilities
RENISCHEM® L-FABP ELISA Kit
-
Q1Is it necessary to dilute samples before measurement?
-
A1Dilution is not always required. However, when L-FABP concentration exceeds 400 ng/mL, please dilute with a Standard Diluent (0 ng/mL), and then re-measure.
-
Q2Is it necessary to adjust urinary L-FABP concentration with urinary creatinine correction?
What is the conversion formula for creatinine correction? -
A2In principle, urinary creatinine correction is necessary to prevent errors in urinary concentration. However, the result obtained with our L-FABP POC kits, determines by L-FABP concentration in urine.
In the case of AKI (Acute Kidney Injury), the urinary creatinine level itself may fluctuate significantly, so the concentration of the urinary L-FABP measurement is also reported, and the actual or corrected value is used at the discretion of the doctor.
Conversion Formula
L-FABP (ug/g・Cr) = L-FABP (ng/mL) ÷ Cr (mg/dL) × 100 -
Q3When is the optimal time for taking urine samples for measurement?
-
A3Urinary L-FABP can be measured in early morning urine, spot urine and pooled urine. However, it is desirable to prepare samples under the same conditions in monitoring L-FABP levels.
-
Q4What are the methods for storing and stabilizing samples?
-
A4Please store samples either by refrigeration (4 degrees Celsius) or freezing (-20 to -30 degrees Celsius, -80 degrees Celsius). Stability has been confirmed for up to 48 hours (2 days) when storing by refrigeration (4 degrees Celsius), and up to one (1) year when storing by freezing (-80 degrees Celsius). When storing samples by refrigeration, please make your measurements within 48 hours.
*If refrigeration is difficult, please use a coolant or similar. -
Q5Is there anything to be careful about in the freezing and thawing of samples?
-
A5Thawing of frozen samples (at -80℃) will not affect the assay value. However, repeated freeze-thaw cycles should be avoided.
*It is recommended to store samples in small portions. After thawing, samples may become cloudy, but measurement is still possible. If the sample is centrifuged and the cloudiness is cleared, it will not affect the assay result. -
Q6Is there any influence on measurement when preservative is added to accumulated urine (samples)?
-
A6Adding hydrochloric acid can sometimes have an effect (values tend to be lower than original L-FABP values) on measurements. The addition of sodium azide or toluene has no effect on measurements.
-
Q7Are there diurnal rhythm variations in urinary L-FABP levels?
-
A7Variations may be observed within the normal range of L-FABP levels.
-
Q8Are there any effects on interference substances?
-
A8Yes. The presence of bilirubin, hemoglobin, glucose or ascorbic acid in urine sample can affect the performance of ELISA Kit. However, the following levels do not influence on measurement value:
- 1. Free bilirubin up to 19.7 mg/dL.
- 2. Conjugated bilirubin up to 21.8 mg/dL.
- 3. Hemolytic hemoglobin up to 24.4 mg/dL.
- 4. Glucose up to 45.0 mg/mL.
- 5. Ascorbic acid up to 12.5 mg/mL.
-
Q9Does the contrast media for angiography have any effect on measurements?
-
A9The timing of clearance of the contrast medium into the urine has no effect on ELISA values (no baseline inhibition). The increase in urinary L-FABP is not due to nephrotoxicity of the contrast agent itself, but rather to a decrease in micro-renal blood flow (renal vasoconstriction) caused by the contrast agent.
RENISCHEM® L-FABP POC Kit
-
Q1Is it necessary to dilute samples before measurement?
-
A1Dilution of samples is not needed.
-
Q2When is the optimal time for taking urine samples for measurement?
-
A2Urinary L-FABP can be measured in early morning urine, spot urine and pooled urine, except for acid pooled urine. However, it is desirable to prepare samples under the same conditions in monitoring L-FABP levels.
-
Q3Which is the optimal sample condition for measurement?
-
A3Urinary L-FABP can be measured fresh urine and stored urine in refrigerator and freezer.
-
Q4How is urine sample stability at room temperature?
-
A4If stored at room temperature up to 8 hours, the effect on the results should be negligible. In rare cases, urine samples containing high concentrations of L-FABP (several hundred ng/mL) have been found to have persistently elevated L-FABP levels, so the package insert recommends that urine samples be measured as soon as possible after collection.
-
Q5Does the foreign substance in the sample have any effect on measurement systems?
-
A5Correct results may not be obtained if the sample is contaminated with foreign matter or is cloudy. Before mixing with the pretreatment, centrifuge or allow to let rest sample in the dark at room temperature for at least 30 minutes and slowly collect 100 µL of specimen from the supernatant to avoid aspiration of foreign matter. The red blood cells (haematuria), cell detachment due to infection or inflammation, and glomerular and tubular tissue shedding can be listed as foreign matters.
-
Q6Can viscous sample be measured?
-
A6Viscous urine samples may not spread through the membrane and may stop or flow at a much slower rate than normal. The RENISCHEM L-FABP ELISA quantification kit is recommended for such samples as it is not possible to obtain correct results.
-
Q7Are there any effects on interference substances?
-
A7Yes. The presence of bilirubin, hemoglobin, glucose or ascorbic acid in urine sample can affect the performance of POC Kit. However, the following levels do not influence on measurement value:
- 1. Free bilirubin up to 19.7 mg/dL.
- 2. Conjugated bilirubin up to 21.8 mg/dL.
- 3. Hemolytic hemoglobin up to 97.4 mg/dL.
- 4. Glucose up to 1000 mg/dL.
- 5. Ascorbic acid up to 1.6 mg/mL.
-
Q8Can colored sample be measured?
-
A8If there is dark coloration on urine sample, it may not give a correct result. For example, if urine from a patient on rifampicin medication is measured with the POC, the entire membrane of POC kits strip will be stained orange, which will be in contrast to the color of the test line and may give a false result. (See pictures below) Hematuria may also stain the membrane. If a correct result is not obtained, it is recommended to re-collect sample at a time when it is not affected by medication and to test with POC kit again or to use RENISCHEM® L-FABP ELISA kit for obtaining quantitative results.

-
Q9Can the sample of patient with end stage renal failure (stage 4 of diabetic nephropathy or end stage kidney disease) be measured?
-
A9Yes, in diabetic nephropathy of type 2 diabetic patients study which consists in measurement according to the different stage of disease(stage 1 to 4), shows that urinary L-FABP levels are correlated with the progression of diabetic nephropathy. So it is possible to measure urinary L-FABP in the stage renal failure. (Diabetes Care 34:691-696, 2011. PubMed▶)
RENISCHEM® L-FABP ELISA Kit
-
Q1What are the details of Human L-FABP ELISA Kit?
-
A1Human L-FABP ELISA Kit is quantitative assay of manual testing. This assay needs 96 well microplate reader for measuring absorbance.
-
Q2What is measurement principle of ELISA method?
-
A2You can browse from the following Measurement principle (ELISA method).
-
Q3How is the component of the Human L-FABP ELISA Kit?
-
A3You can browse from the following Product composition.
-
Q4What is the recommended storage condition and shelf-life for Human
L-FABP ELISA Kit? -
A4Storage condition: between 2-8°C (avoid freezing)
Shelf-life: 24 months (expiry date is written on outer case) -
Q5Is it possible to obtain instruction for use?
-
A5You can browse from the following Instruction.
-
Q6How much does the product cost?
-
A6About product cost, please Contact us.
-
Q7Is this product returnable?
-
A7We will not accept your return request due to the nature of product.
RENISCHEM® L-FABP POC Kit
-
Q1What is RENISCHEM® L-FABP POC Kit?
-
A1RENISCHEM® L-FABP POC Kit is semi-quantitative assay of manual testing. This assay is used at bedside without any reader devices.
-
Q2What is the meaning of POC and the measurement principle?
-
A2POC is an abbreviation for “Point of Care”, and can be performed at the bedside. This test is the rapid test which uses immunoadsorbent chromatography measurement method, similar to the flu kits. About measurement principle, you can browse from the following Measurement principle (POC).
-
Q3What is the recommended storage condition and shelf-life for RENISCHEM® L-FABP POC Kit?
-
A3Storage condition: between 1-30°C (avoid freezing)
Shelf-life: 18 months (expiry date is written on outer case) -
Q4Is it possible to obtain instruction for use?
-
A4You can browse from the following Instruction.
-
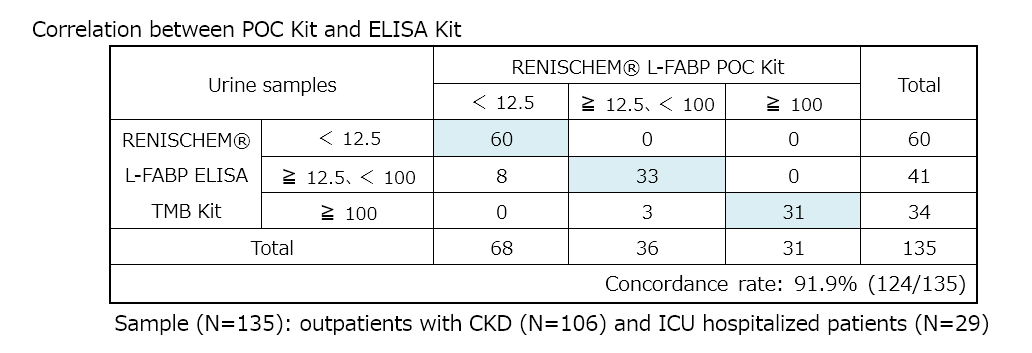
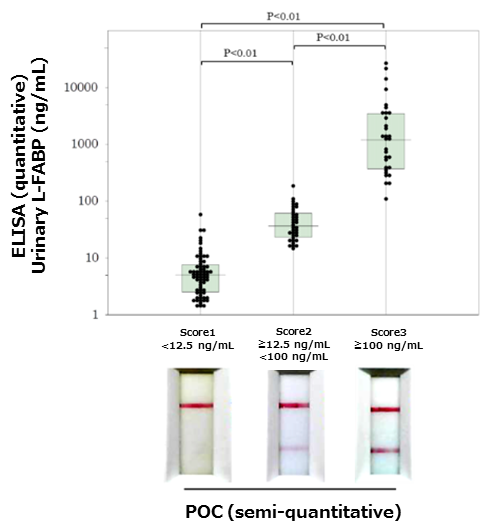
Q5Is there correlation between semi-quantitative POC Kit and quantitative ELISA Kit?
-
A5High concordance rate over 90% is calculated from the measurement values of POC Kit and ELISA Kit.
-
Q6What is product cost and distributor?
-
A6About product cost and distributor, please Contact us.
-
Q7Is this product returnable?
-
A7We will not accept your return request due to the nature of product.
-
Q1Are there any recommendations of renal injury markers to be compared with L-FABP?
-
A1In case that you measure glomerular injury marker in combination with L-FABP as a tubular injury marker, the renal injury level can be evaluated.
-
Q2Q2. Can we use human renal biopsy as the sample?
-
A2If you collect the supernatant from appropriate amount of tissue homogenate, you can use the supernatant with pretreatment as the sample for ELISA Kit. However, we will not be able to guarantee the performance of our product because the original purpose of the product is to measure urinary L-FABP.
-
Q3Can we measure blood L-FABP level?
-
A3You can measure L-FABP level within the blood. However, we will not be able to guarantee the performance of our product because the original purpose of the product is to measure urinary L-FABP. In addition, we do not have information about the stability and the cut-off value of L-FABP in blood.